Research Interests
| (We appreciate professor Yan Xu for providing the image.) In addition to the well-known DNA double-helix, nucleic acids are prone to structural polymorphism. In fact, a number of non-B DNA structures, which may related to genetic diseases, are proved recently, and widely used in nanotechnology, supramolecular chemistry, biosensors et al (Chem. Soc. Rev., 2011, 40, 2719–2740; 5293–5307; 5867–5892; 5893–5909; 2013, 42, 3427–3440; 2014, 43, 518–529; 2016, 45, 3188–3206). For example, guanine rich sequences can form higher order nucleic acid secondary structure, a well-known molecule G-quadruplex (G4) (Chem. Soc. Rev., 2008, 37, 1375–1384). G4 has emerged as more than a novelty. Similar to other unusual DNA forms, it not only shows great potential in non-bio applications (Chem.Rev., 2013, 113, 3044–3083; Angew. Chem. Int. Ed., 2015, 54, 1098–1129; 12212–12235et al), but also exhibits regulatory role and therapeutic potential in human (Nat. Rev. Genet., 2012, 13, 770–780; Nucleic Acids Res.,2015, 43, 8627–8637; Nat. Rev. Mol. Cell. Biol., 2017, 18, 279–284 et al). G4 is an expanding field, more and more researchers are devoting to the glamorous structures, and they gather to multi-aspect demonstrate their charming progress biennially (2013, http://www.g4meeting2013.com/; 2015, http://g4meeting2015.sciencesconf.org/; 2017, www.g4thering.cz; 2019,http://g4meeting2019.csp.escience.cn/dct/page/1; Previous three conferences(2007, 2009, and 2011) do not have websites). We are the new-emerging force in G4, and focus on its structure, properties, and applications (representative works show below), and hope others to collaborate with us in future. |
Non-classical DNA structure
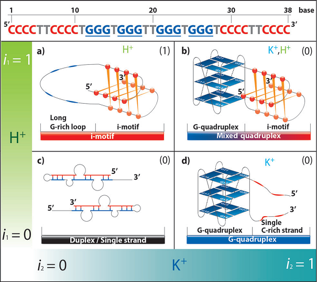
Except G-quadruplex, its complementary cytosine rich sequences, also can form quadruplex structure, called i-motif. These two sequences are easy to form duplex under physiologicalconditions. We constructed a mixed quadruplex, that is G-quadruplex and i-motif coexisted inthe single strand, and found that these structures can be tuned by environment conditions, such as pH and cations. More importantly, the structural transition is very fast, which can beused as NOTIF logical gates.
G-quadruplex

|
Unexpected Position-Dependent Effects of Ribose G‑Quartets in G‑Quadruplexes To understand the role of ribose G-quartets and how they affect the properties of G-quadruplex structures, we studied three systems in which one, two, three, or four deoxyribose G-quartets were substituted withribose G-quartets. These systems were a parallel DNA intramolecular G-quadruplex,d(TTGGGTGGGTTGGGTGGGTT), and two tetramolecular G-quadruplexes, d(TGGGT) and d(TGGGGT).Thermal denaturation experiments revealed that ribose G-quartets have position-dependent andcumulative effects
on G-quadruplex stability. An unexpected destabilization was observed
when rG quartets were presented at the 5′-end of the G stack. This
observation challenges the general belief that RNAresidues stabilize G-quadruplexes. Furthermore, in contrast to past proposals, hydration is not the mainfactor
determining the stability of our RNA/DNA chimeric G-quadruplexes.
Interestingly, the presence of rG residues in a central G-quartet
facilitated the formation of additional tetramolecular G-quadruplex
topolo-gies showing positive circular dichroism signals at 295 nm. 2D
NMR analysis of the tetramolecular TGgGGT (lowercase letter indicates
ribose) indicates that Gs in the 5′-most G-quartet adopt the syn
conformation.These
analyses highlight several new aspects of the role of ribose G-quartets
on G-quadruplex structure andstability, and demonstrate that the
positions of ribose residues are critical for tuning G-quadruplexproperties. |
G-quadruplex/hemin DNAzyme
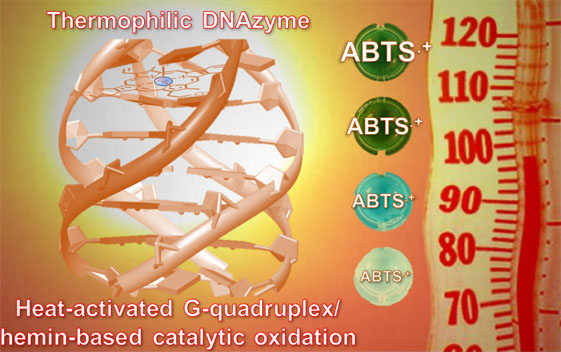
| A Thermophilic TetramolecularG-quadruplex/Hemin DNAzymeQuadruplex-based DNAzyme system is one of the most useful artificial enzymes or catalysts due to theirunique properties that make them reliable alternatives to proteins for performing catalytic transformation. Herein, we report on the first prototypeof a thermally stable DNAzyme system, referred to as a thermophilicDNAzyme, capable of oxidizing substrates in harsh experimental conditions, i.e., high temperature (up to 95 °C) and long reaction times (up to 18 h at 75 °C). The step-by-step design of this unique heat-activatedG-quadruplex/hemin catalyst, including the modification of adenines at both ends of G-tracts, the choice ofcation and its concentration for DNAzyme stabilization, is described. This work investigates thoroughly themolecular basis of these catalytic properties and provides an example of an industrially relevant application. |